| Identification |
| Name | Idelalisib, CAS 870281-82-6 |
| Structure | 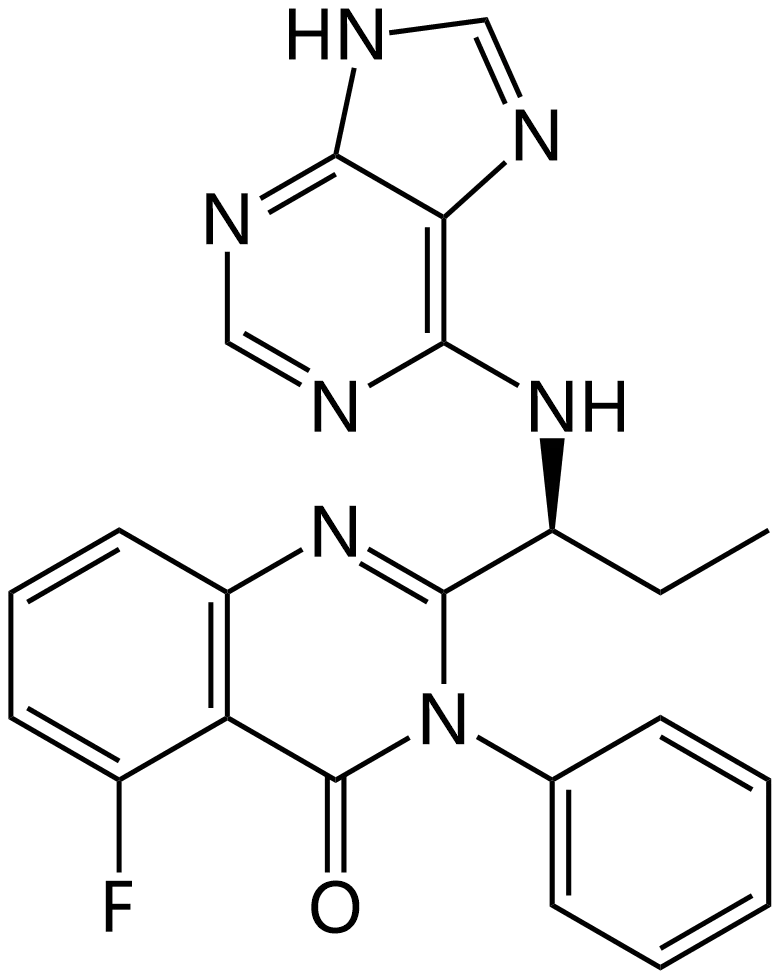 |
| Other name | Idelalisib 870281-82-6 CAL-101 GS-1101 CAL 101 CAL101 (S)-2-(1-((9H-Purin-6-yl)amino)propyl)-5-fluoro-3-phenylquinazolin-4(3H)-one UNII-YG57I8T5M0 CAL-101 (Idelalisib, GS-1101) |
| CAS NO. | 870281-82-6 |
| Molecular Formula | C22H18FN7O |
| Molecular Weight | 415.4 g/mol |
| Chemical and Physical Properties | |
| Appearance | White crystalline powder |
| Quality standard | Purity 99% min |
| Application | |
| Drug Indication | Idelalisib is indicated in the treatment of chronic lymphocytic leukemia (CLL), relapsed follicular B-cell non-Hodgkin lymphoma (FL), and relapsed small lymphocytic lymphoma (SLL). For the treatment of relapsed CLL, it is currently indicated as a second-line agent in combination with rituximab in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities, while in the treatment of FL and SLL it is intended to be used in patients who have received at least two prior systemic therapies. |
| Formulations/Preparations | Tablet |
|
Handling and storage |
|
| Safety and Hazards | The shanghai Research Institute of Chemical industry testing center identification conclusion :1 None hazard identification, 2 The substance is not subject to IATA DGR, 3 Non packing requirements. |
| Handling and storage | Keep container tightly closed in a dry and well-ventilated place. Light sensitive. |
| Sample package | Aluminium foil bag |
| Commercial package | Aluminium foil bag |
| UN Code | Not classfied |
| HS Code | 29399990 |
| Shipping group | Shipment at ambient temperature for no controlled and no dangerous goods |
| Sales restriction | No |
| Origin | China |
| Technical Support | |
| Idelalisib STD Data sheet | Yes |
| Idelalisib MSDS | Yes |
| Idelalisib COA | Yes |
| Idelalisib Transport Certificate | Yes |
| Supply ability | |
| Monthly capacity | 10kg |
| GMP | None |
| Related APIs | |
| Related APIS | NVP-BKM120 IC-87114 GDC-0941 Show all 8 |
